What we do
Apps & Software
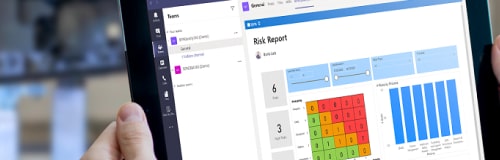
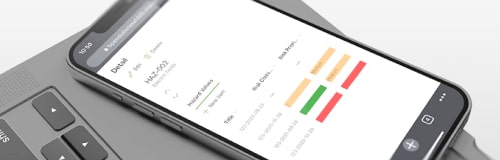
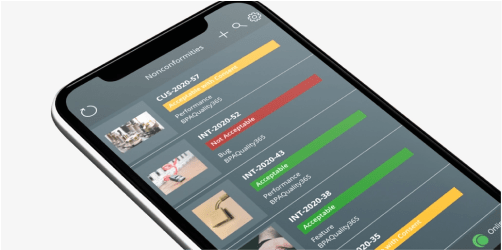
Quality & Risk Management
Reach smart quality and enable the future of work with BPAQuality365®.
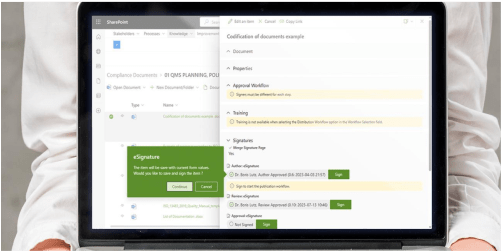
Medical Compliance
BPAMedical365® helps you to tackle ISO 13485, MDR and FDA regulations with ease.
Solutions
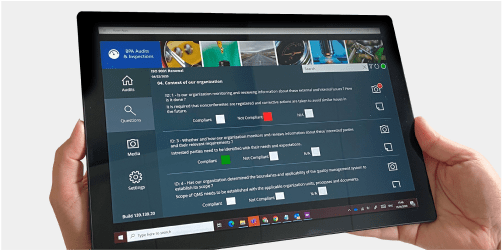
WHY MICROSOFT 365



Profitable
With Microsoft 365, clients save significant costs by eliminating redundant solutions, simplifying IT management, and increasing security of digital workers.
Our clients








What’s New

Shift to ultimate quality management with BPA Premium edition
We are proud to announce the next-coming release of our eQMS Premium Edition...

Revolutionize audit management with BPA and GenAI
Auditing the QMS is essential to ensure all processes are efficient to deliver...
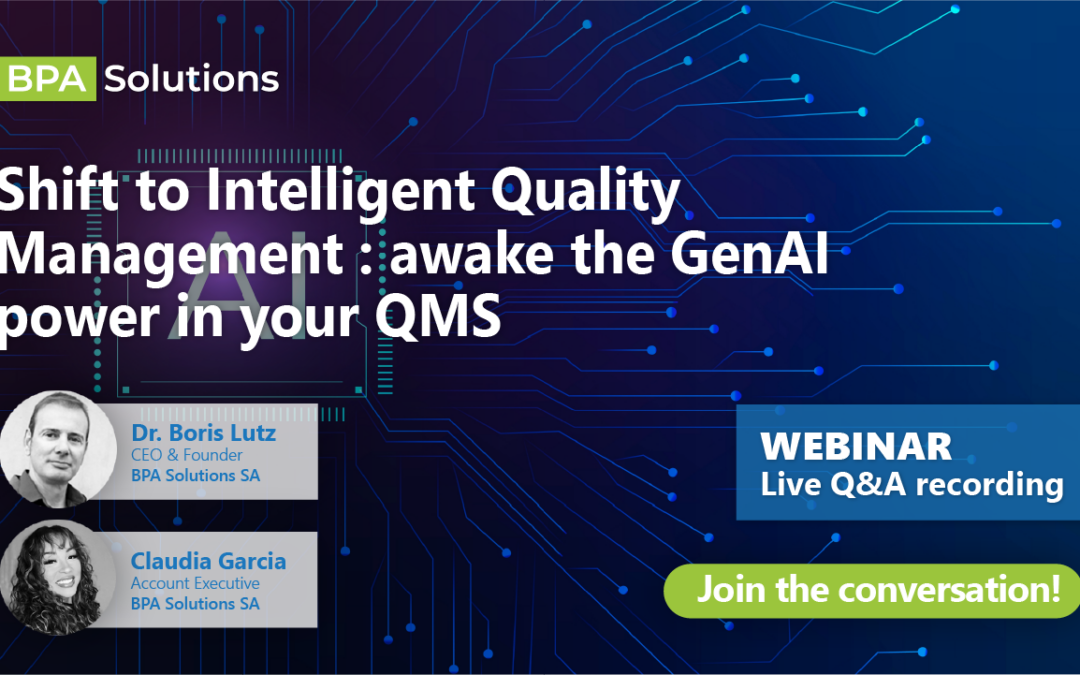
Unlock Intelligent Quality: insights from our webinar
This month we had our second Webinar uncovering how BPA Pilot, our newly...